
Last update: 02/07/2019
For all high throughput sequencing applications, we would recommend performing some quality control on the data, as it can often straight away point you towards the next steps that need to be taken (e.g. with FastQC). Thorough quality control and taking appropriate steps to remove problems is vital for the analysis of almost all sequencing applications. This is even more critical for the proper analysis of RRBS libraries since they are susceptible to a variety of errors or biases that one could probably get away with in other sequencing applications. In our brief guide to RRBS we discuss the following points:
Poor base call qualities or adapter contamination are however just as relevant for ‘normal’, i.e. non-RRBS, libraries.
We have tried to implement a method to rid RRBS libraries (or other kinds of sequencing datasets) of potential problems in one convenient process. For this we have developed a wrapper script (trim_galore) that makes use of the publicly available adapter trimming tool Cutadapt and FastQC for optional quality control once the trimming process has completed.
Even though Trim Galore works for any (base space) high throughput dataset (e.g. downloaded from the SRA) this section describes its use mainly with respect to RRBS libraries.
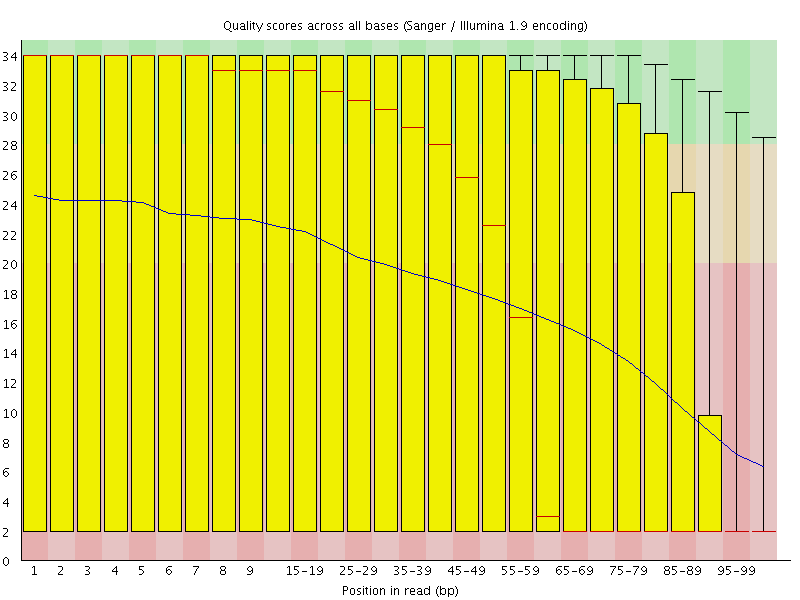
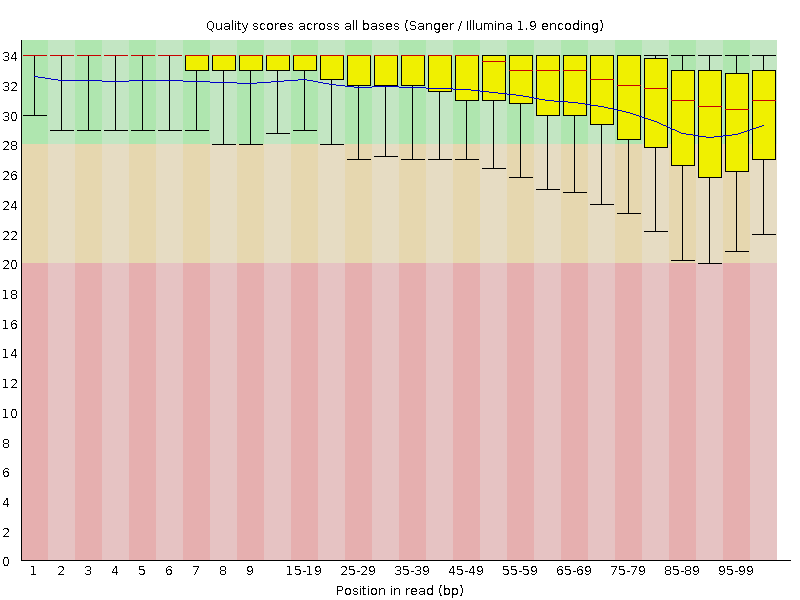
In the first step, low-quality base calls are trimmed off from the 3’ end of the reads before adapter removal. This efficiently removes poor quality portions of the reads.
 |
 |
|---|---|
| Before Quality Trimming | After Quality Trimming |
 |
 |
Here is an example of a dataset downloaded from the SRA which was trimmed with a Phred score threshold of 20 (data set DRR001650_1 from Kobayashi et al., 2012).
In the next step, Cutadapt finds and removes adapter sequences from the 3’ end of reads.
If no sequence was supplied, Trim Galore will attempt to auto-detect the adapter which has been used. For this it will analyse the first 1 million sequences of the first specified file and attempt to find the first 12 or 13bp of the following standard adapters:
Illumina: AGATCGGAAGAGC
Small RNA: TGGAATTCTCGG
Nextera: CTGTCTCTTATAIf no adapter contamination can be detected within the first 1 million sequences, or in case of a tie between several different adapters, Trim Galore defaults to --illumina, as long as the Illumina adapter sequence was one of the options. If there was a tie between the Nextera and small RNA adapter, the default is --nextera. The auto-detection results are shown on screen and printed to the trimming report for future reference.
The auto-detection behaviour can be overruled by specifying an adapter sequence manually or by using --illumina, --nextera or --small_rna. Please note: the first 13 bp of the standard Illumina paired-end adapters (AGATCGGAAGAGC) recognise and removes adapter from most standard libraries, including the Illumina TruSeq and Sanger iTag adapters. This sequence is present on both sides of paired-end sequences, and is present in all adapters before the unique Index sequence occurs. So for any ‘normal’ kind of sequencing you do not need to specify anything but --illumina, or better yet just use the auto-detection.
To control the stringency of the adapter removal process one gets to specify the minimum number of required overlap with the adapter sequence; else it will default to 1. This default setting is extremely stringent, i.e. an overlap with the adapter sequence of even a single bp is spotted and removed. This may appear unnecessarily harsh; however, as a reminder adapter contamination may in a Bisulfite-Seq setting lead to mis-alignments and hence incorrect methylation calls, or result in the removal of the sequence as a whole because of too many mismatches in the alignment process.
Tolerating adapter contamination is most likely detrimental to the results, but we realize that this process may in some cases also remove some genuine genomic sequence. It is unlikely that the removed bits of sequence would have been involved in methylation calling anyway (since only the 4th and 5th adapter base would possibly be involved in methylation calls, for directional libraries). However, it is quite likely that true adapter contamination – irrespective of its length – would be detrimental for the alignment or methylation call process, or both.
| Before Adapter Trimming | After Adapter Trimming |
|---|---|
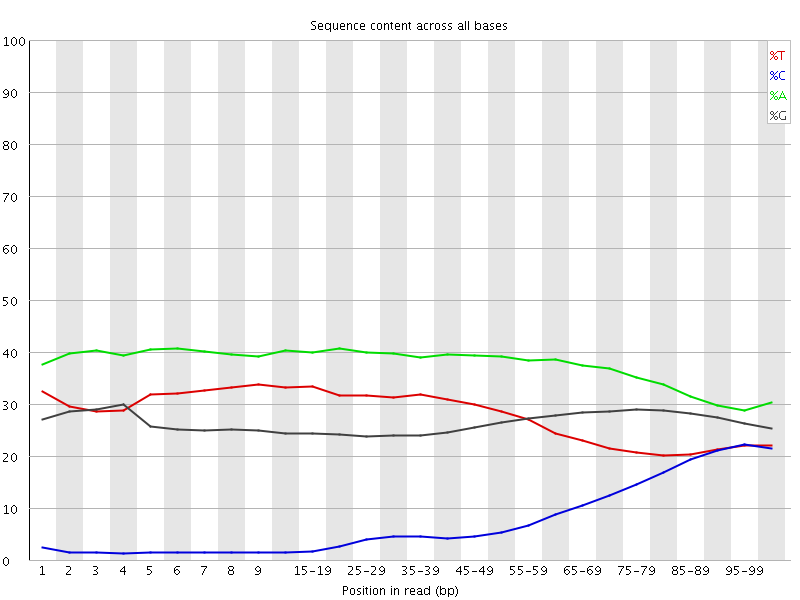 |
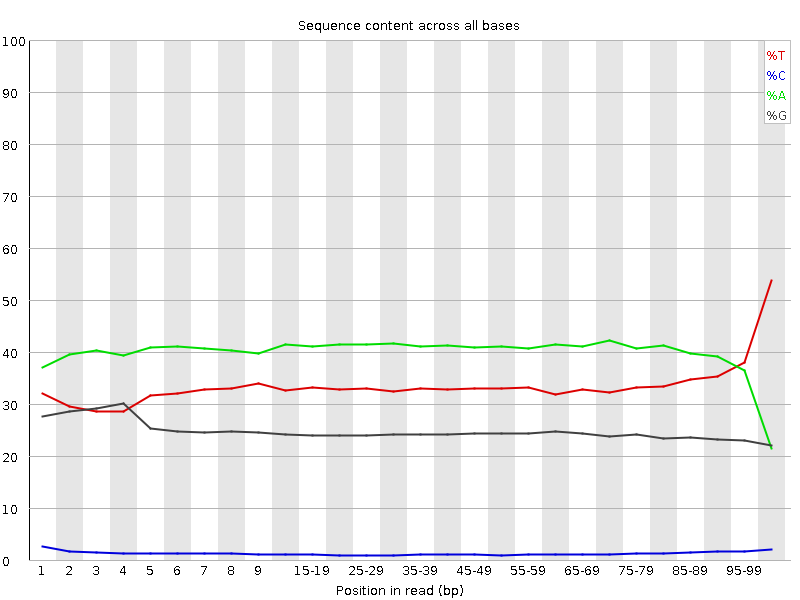 |
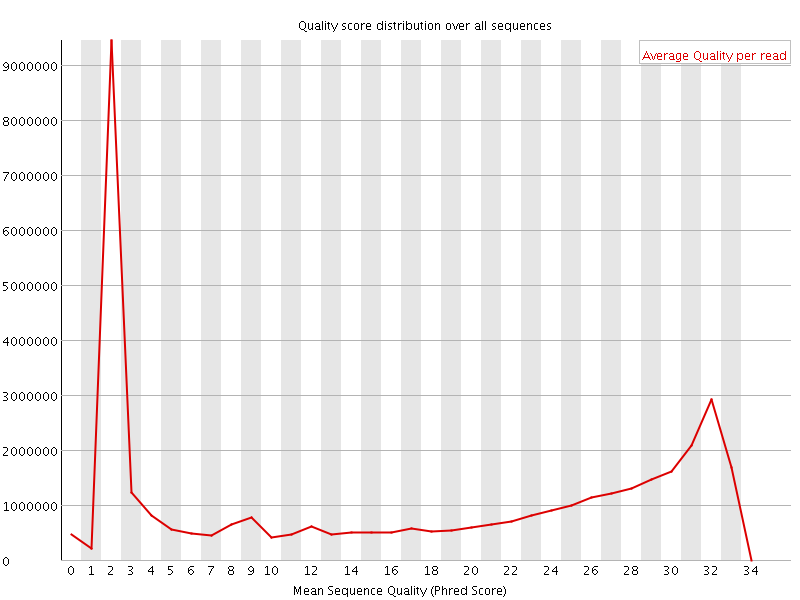
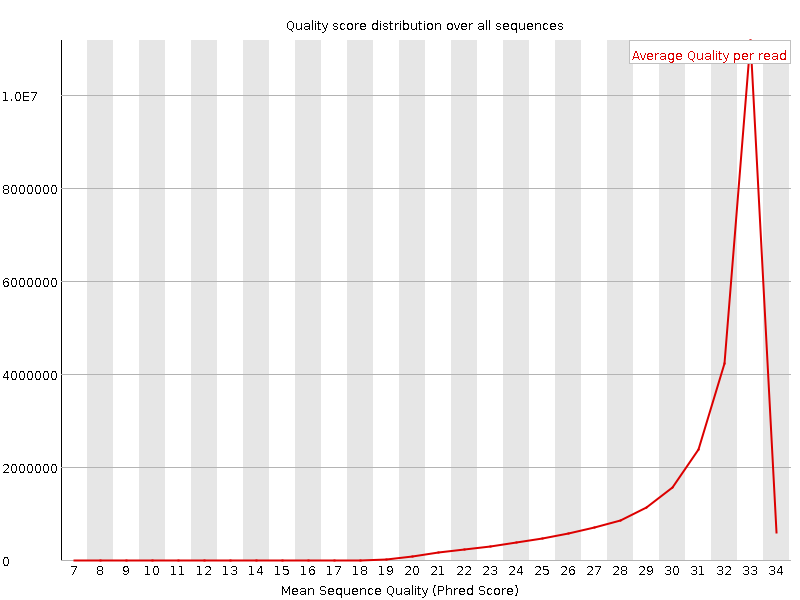
This example (same dataset as above) shows the dramatic effect of adapter contamination on the base composition of the analysed library, e.g. the C content rises from ~1% at the start of reads to around 22% (!) towards the end of reads. Adapter trimming with Cutadapt gets rid of most signs of adapter contamination efficiently. Note that the sharp decrease of A at the last position is a result of removing the adapter sequence very stringently, i.e. even a single trailing A at the end is removed.
Trim galore! also has an --rrbs option for DNA material that was digested with the restriction enzyme MspI. In this mode, Trim Galore identifies sequences that were adapter-trimmed and removes another 2 bp from the 3’ end of Read 1, and for paired-end libraries also the first 2 bp of Read 2 (which is equally affected by the fill-in procedure). This is to avoid that the filled-in cytosine position close to the second MspI site in a sequence is used for methylation calls. Sequences which were merely trimmed because of poor quality will not be shortened any further.
Trim Galore! also has a --non_directional option, which will screen adapter-trimmed sequences for the presence of either CAA or CGA at the start of sequences and clip off the first 2 bases if found. If CAA or CGA are found at the start, no bases will be trimmed off from the 3’ end even if the sequence had some contaminating adapter sequence removed (in this case the sequence read likely originated from either the CTOT or CTOB strand; refer to the RRBS guide for the meaning of CTOT and CTOB strands).
Lastly, since quality and/or adapter trimming may result in very short sequences (sometimes as short as 0 bp), Trim Galore! can filter trimmed reads based on their sequence length (default: 20 bp). This is to reduce the size of the output file and to avoid crashes of alignment programs which require sequences with a certain minimum length.
Note that it is not recommended to remove too-short sequences if the analysed FastQ file is one of a pair of paired-end files, since this confuses the sequence-by-sequence order of paired-end reads which is again required by many aligners. For paired-end files, Trim Galore! has an option --paired which runs a paired-end validation on both trimmed _1 and _2 FastQ files once the trimming has completed. This step removes entire read pairs if at least one of the two sequences became shorter than a certain threshold. If only one of the two reads is longer than the set threshold, e.g. when one read has very poor qualities throughout, this singleton read can be written out to unpaired files (see option retain_unpaired) which may be aligned in a single-end manner.
Applying these steps to both self-generated and downloaded data can ensure that you really only use the high quality portion of the data for alignments and further downstream analyses and conclusions.
The option --hardtrim5 INT allows you to hard-clip sequences from their 3’ end. This option processes one or more files (plain FastQ or gzip compressed files) and produces hard-trimmed FastQ files ending in .{INT}bp_5prime.fq(.gz). This is useful when you want to shorten reads to a certain read length. Here is an example:
before: CCTAAGGAAACAAGTACACTCCACACATGCATAAAGGAAATCAAATGTTATTTTTAAGAAAATGGAAAAT
--hardtrim5 20: CCTAAGGAAACAAGTACACTThe option --hardtrim3 INT allows you to hard-clip sequences from their 5’ end. This option processes one or more files (plain FastQ or gzip compressed files) and produces hard-trimmed FastQ files ending in .{INT}bp_3prime.fq(.gz). We found this quite useful in a number of scenarios where we wanted to remove biased residues from the start of sequences. Here is an example :
before: CCTAAGGAAACAAGTACACTCCACACATGCATAAAGGAAATCAAATGTTATTTTTAAGAAAATGGAAAAT
--hardtrim3 20: TTTTTAAGAAAATGGAAAATThe option --clock trims reads in a specific way that is currently used for the Mouse Epigenetic Clock (see here: Multi-tissue DNA methylation age predictor in mouse, Stubbs et al., Genome Biology, 2017 18:68). Following the trimming, Trim Galore exits.
In it’s current implementation, the dual-UMI RRBS reads come in the following format:
Read 1 5' UUUUUUUU CAGTA FFFFFFFFFFFFFFFFFFFFFFFFFFFFFFFFFFFF TACTG UUUUUUUU 3'
Read 2 3' UUUUUUUU GTCAT FFFFFFFFFFFFFFFFFFFFFFFFFFFFFFFFFFFF ATGAC UUUUUUUU 5'Where UUUUUUUU is a random 8-mer unique molecular identifier (UMI), CAGTA is a constant region, and FFFFFFF… is the actual RRBS-Fragment to be sequenced. The UMIs for Read 1 (R1) and Read 2 (R2), as well as the fixed sequences (F1 or F2), are written into the read ID and removed from the actual sequence. Here is an example:
R1: @HWI-D00436:407:CCAETANXX:1:1101:4105:1905 1:N:0: CGATGTTT
ATCTAGTTCAGTACGGTGTTTTCGAATTAGAAAAATATGTATAGAGGAAATAGATATAAAGGCGTATTCGTTATTG
R2: @HWI-D00436:407:CCAETANXX:1:1101:4105:1905 3:N:0: CGATGTTT
CAATTTTGCAGTACAAAAATAATACCTCCTCTATTTATCCAAAATCACAAAAAACCACCCACTTAACTTTCCCTAA
R1: @HWI-D00436:407:CCAETANXX:1:1101:4105:1905 1:N:0: CGATGTTT:R1:ATCTAGTT:R2:CAATTTTG:F1:CAGT:F2:CAGT
CGGTGTTTTCGAATTAGAAAAATATGTATAGAGGAAATAGATATAAAGGCGTATTCGTTATTG
R2: @HWI-D00436:407:CCAETANXX:1:1101:4105:1905 3:N:0: CGATGTTT:R1:ATCTAGTT:R2:CAATTTTG:F1:CAGT:F2:CAGT
CAAAAATAATACCTCCTCTATTTATCCAAAATCACAAAAAACCACCCACTTAACTTTCCCTAAFollowing clock trimming, the resulting files (.clock_UMI.R1.fq(.gz) and .clock_UMI.R2.fq(.gz)) should be adapter- and quality trimmed with a second Trim Galore run. Even though the data is technically RRBS, it doesn’t require the --rrbs option. Instead the reads need to be trimmed by 15bp from their 3’ end to get rid of potential UMI and fixed sequences. All this is accomplished with this additional trimming command:
trim_galore --paired --three_prime_clip_R1 15 --three_prime_clip_R2 15 *.clock_UMI.R1.fq.gz *.clock_UMI.R2.fq.gz
Following this, reads should be aligned with Bismark and deduplicated with UmiBam in --dual_index mode (see here: https://github.com/FelixKrueger/Umi-Grinder). UmiBam recognises the UMIs within this pattern: R1:(ATCTAGTT):R2:(CAATTTTG): as (UMI R1=ATCTAGTT) and (UMI R2=CAATTTTG).
USAGE: trim_galore [options] <filename(s)>
-h/--help
-v/--version
-q/--quality <INT>
20--phred33
ASCII+33 quality scores as Phred scores (Sanger/Illumina 1.9+ encoding) for quality trimming.ON--phred64
ASCII+64 quality scores as Phred scores (Illumina 1.5 encoding) for quality trimming.--fastqc
--fastqc_args "<ARGS>"
arg1 arg2 [..].--fastqc_args "--nogroup --outdir /home/".--fastqc does not have to be specified separately.-a/--adapter <STRING>
--illumina, --nextera and --small_rna.--illumina. A single base may also be given as e.g. -a A{10}, to be expanded to -a AAAAAAAAAA.-a2/--adapter2 <STRING>
--paired to be specified as well. If the libraries to be trimmed are smallRNA then a2 will be set to the Illumina small RNA 5’ adapter automatically (GATCGTCGGACT). A single base may also be given as e.g. -a2 A{10}, to be expanded to -a2 AAAAAAAAAA.--illumina
AGATCGGAAGAGC instead of the default auto-detection of adapter sequence.--nextera
CTGTCTCTTATA instead of the default auto-detection of adapter sequence.--small_rna
TGGAATTCTCGG instead of the default auto-detection of adapter sequence.--length value to 18bp. If the smallRNA libraries are paired-end then -a2 will be set to the Illumina small RNA 5’ adapter automatically (GATCGTCGGACT) unless -a 2 had been defined explicitly.--max_length <INT>
--stringency <INT>
1, i.e. even a single base pair of overlapping sequence will be trimmed of the 3’ end of any read.-e <ERROR RATE>
0.1--gzip
gzip.--dont_gzip
--gzip.--length <INT>
0 effectively disables this behaviour.20 bp.--paired). If only one read became too short there is the possibility of keeping such unpaired single-end reads (see --retain_unpaired).20 bp.--max_n COUNT
Ns (as integer) a read may contain before it will be removed altogether.--trim-n
Ns from either side of the read.-o/--output_dir <DIR>
--no_report_file
--suppress_warn
STDOUT or STDERR will be suppressed.--clip_R1 <int>
OFF--clip_R2 <int>
OFF--three_prime_clip_R1 <int>
<int> bp from the 3’ end of read 1 (or single-end reads) AFTER adapter/quality trimming has been performed. This may remove some unwanted bias from the 3’ end that is not directly related to adapter sequence or basecall quality.OFF--three_prime_clip_R2 <int>
<int> bp from the 3’ end of read 2 AFTER adapter/quality trimming has been performed. This may remove some unwanted bias from the 3’ end that is not directly related to adapter sequence or basecall quality.OFF--2colour/--nextseq INT
--nextseq-trim=3'CUTOFF within Cutadapt, which will set a quality cutoff (that is normally given with -q instead), but qualities of G bases are ignored. This trimming is in common for the NextSeq- and NovaSeq-platforms, where basecalls without any signal are called as high-quality G bases. More on the issue of G-overcalling may be found here: https://sequencing.qcfail.com/articles/illumina-2-colour-chemistry-can-overcall-high-confidence-g-bases/. This is mutually exlusive with -q INT.--path_to_cutadapt </path/to/cutadapt>
/my/home/cutadapt-1.7.1/bin/cutadapt. Else it is assumed that Cutadapt is in the PATH.--basename <PREFERRED_NAME>
PREFERRED_NAME_trimmed.fq(.gz), or PREFERRED_NAME_val_1.fq(.gz) and PREFERRED_NAME_val_2.fq(.gz) for paired-end data. --basename only works when 1 file (single-end) or 2 files (paired-end) are specified, but not for longer lists.-j/--cores INT
Number of cores to be used for trimming [default: 1]. For Cutadapt to work with multiple cores, it requires Python 3 as well as parallel gzip (pigz) installed on the system. The version of Python used is detected from the shebang line of the Cutadapt executable (either cutadapt, or a specified path). If Python 2 is detected, --cores is set to 1. If pigz cannot be detected on your system, Trim Galore reverts to using gzip compression. Please note that gzip compression will slow down multi-core processes so much that it is hardly worthwhile, please see: https://github.com/FelixKrueger/TrimGalore/issues/16#issuecomment-458557103 for more info).
Actual core usage: It should be mentioned that the actual number of cores used is a little convoluted. Assuming that Python 3 is used and pigz is installed, --cores 2 would use 2 cores to read the input (probably not at a high usage though), 2 cores to write to the output (at moderately high usage), and 2 cores for Cutadapt itself + 2 additional cores for Cutadapt (not sure what they are used for) + 1 core for Trim Galore itself. So this can be up to 9 cores, even though most of them won’t be used at 100% for most of the time. Paired-end processing uses twice as many cores for the validation (= writing out) step. --cores 4 would then be: 4 (read) + 4 (write) + 4 (Cutadapt) + 2 (extra Cutadapt) + 1 (Trim Galore) = 15, and so forth.
It seems that --cores 4 could be a sweet spot, anything above has diminishing returns.
--hardtrim5 <int>
.<int>bp_5prime.fq(.gz).--hardtrim3 <int>
.<int>bp_3prime.fq(.gz).--clock
In it’s current implementation, the dual-UMI RRBS reads come in the following format:
Read 1 5' UUUUUUUU CAGTA FFFFFFFFFFFFFFFFFFFFFFFFFFFFFFFFFFFF TACTG UUUUUUUU 3'
Read 2 3' UUUUUUUU GTCAT FFFFFFFFFFFFFFFFFFFFFFFFFFFFFFFFFFFF ATGAC UUUUUUUU 5'Where UUUUUUUU is a random 8-mer unique molecular identifier (UMI), CAGTA is a constant region and FFFFFFF… is the actual RRBS-Fragment to be sequenced. The UMIs for Read 1 (R1) and Read 2 (R2), as well as the fixed sequences (F1 or F2), are written into the read ID and removed from the actual sequence. Here is an example:
R1: @HWI-D00436:407:CCAETANXX:1:1101:4105:1905 1:N:0: CGATGTTT
ATCTAGTTCAGTACGGTGTTTTCGAATTAGAAAAATATGTATAGAGGAAATAGATATAAAGGCGTATTCGTTATTG
R2: @HWI-D00436:407:CCAETANXX:1:1101:4105:1905 3:N:0: CGATGTTT
CAATTTTGCAGTACAAAAATAATACCTCCTCTATTTATCCAAAATCACAAAAAACCACCCACTTAACTTTCCCTAA
R1: @HWI-D00436:407:CCAETANXX:1:1101:4105:1905 1:N:0: CGATGTTT:R1:ATCTAGTT:R2:CAATTTTG:F1:CAGT:F2:CAGT
CGGTGTTTTCGAATTAGAAAAATATGTATAGAGGAAATAGATATAAAGGCGTATTCGTTATTG
R2: @HWI-D00436:407:CCAETANXX:1:1101:4105:1905 3:N:0: CGATGTTT:R1:ATCTAGTT:R2:CAATTTTG:F1:CAGT:F2:CAGT
CAAAAATAATACCTCCTCTATTTATCCAAAATCACAAAAAACCACCCACTTAACTTTCCCTAAFollowing clock trimming, the resulting files (.clock_UMI.R1.fq(.gz) and .clock_UMI.R2.fq(.gz)) should be adapter- and quality trimmed with Trim Galore as usual. In addition, reads need to be trimmed by 15bp from their 3’ end to get rid of potential UMI and fixed sequences. The command is:
trim_galore --paired --three_prime_clip_R1 15 --three_prime_clip_R2 15 *.clock_UMI.R1.fq.gz *.clock_UMI.R2.fq.gzFollowing this, reads should be aligned with Bismark and deduplicated with UmiBam in --dual_index mode (see here: https://github.com/FelixKrueger/Umi-Grinder). UmiBam recognises the UMIs within this pattern: R1:(ATCTAGTT):R2:(CAATTTTG): as (UMI R1=ATCTAGTT) and (UMI R2=CAATTTTG).
--rrbs
CCGG). Sequences which were adapter-trimmed will have a further 2 bp removed from their 3’ end. This is to avoid that the filled-in C close to the second MspI site in a sequence is used for methylation calls. Sequences which were merely trimmed because of poor quality will not be shortened further.--non_directional
CAA or CGA at the start of the read and, if found, removes the first two base pairs. Like with the option --rrbs this avoids using cytosine positions that were filled-in during the end-repair step. --non_directional requires --rrbs to be specified as well.--keep
OFFIf your DNA material was digested with MseI (recognition motif: TTAA) instead of MspI it is NOT necessary to specify --rrbs or --non_directional since virtually all reads should start with the sequence TAA, and this holds true for both directional and non-directional libraries. As the end-repair of TAA restricted sites does not involve any cytosines it does not need to be treated especially. Instead, simply run Trim Galore! in the standard, i.e. non-RRBS, mode.
--paired
--length (see above). If only one read passes this length threshold the other read can be rescued (see option --retain_unpaired).file1_1.fq file1_2.fq SRR2_1.fq.gz SRR2_2.fq.gz ... .-t/--trim1
R1 --------------------------->
R2 <---------------------------
# or this:
R1 ----------------------->
R2 <-------------------retain_unpaired
.unpaired_1.fq or .unpaired_2.fq output files. The length cutoff for unpaired single-end reads is governed by the parameters -r1/--length_1 and -r2/--length_2.OFF.-r1/--length_1 <INT>
.unpaired_1.fq output file. These reads may be mapped in single-end mode.35 bp-r2/--length_2 <INT>
.unpaired_2.fq output file. These reads may be mapped in single-end mode.35 bp